Introduction
Metals are usually extracted from ores through the application of a considerable amount of energy.
Corrosion is the natural disintegration of the refined, pure metal back to the more chemically-stable form,
such as its oxide, hydroxide, or sulphide.
This occurs through an unintended chemical or electrochemical reaction with their environment.
These corrosion processes have the essential features of a battery.
The driving force behind corrosion is a more or less pronounced tendency of metals to be oxidized.
A tabulation of the relative strength of this tendency is called galvanic series.
Metals with low oxidation tendency such as gold are called noble, whereas metals with high
oxidation tendency such as zinc can be used to inhibit or reduce the oxidation process of a more noble metal.
Corrosion
 Corrosion is the primary means by which metals deteriorate.
Most metals corrode on contact with water (and moisture in the air),
acids, bases, salts, oils, aggressive metal polishes, and other solid
and liquid chemicals.
Metals will also corrode when exposed to gaseous materials like acid vapours,
formaldehyde gas, ammonia gas, and sulphur containing gases.
Corrosion specifically refers to any process involving the deterioration
or degradation of metal components. The best known case is that of the
rusting of steel.
Corrosion is the primary means by which metals deteriorate.
Most metals corrode on contact with water (and moisture in the air),
acids, bases, salts, oils, aggressive metal polishes, and other solid
and liquid chemicals.
Metals will also corrode when exposed to gaseous materials like acid vapours,
formaldehyde gas, ammonia gas, and sulphur containing gases.
Corrosion specifically refers to any process involving the deterioration
or degradation of metal components. The best known case is that of the
rusting of steel.
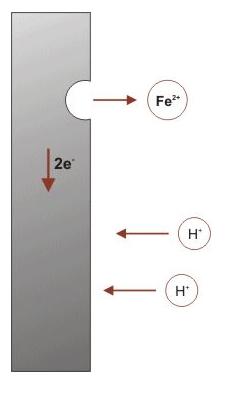
When metal atoms are exposed to an environment containing water molecules
they can give up electrons, becoming themselves positively charged ions,
provided an electrical circuit can be completed.
This effect can be concentrated locally to form a pit or, sometimes a crack,
or it can extend across a wide area to produce general wastage.
Localized corrosion that leads to pitting may provide sites for fatigue
initiation and, additionally, corrosive agents like seawater may lead to
greatly enhanced growth of the fatigue crack. Pitting corrosion also occurs
much faster in areas where micro-structural changes have occurred due to
welding operations.
The corrosion process (anodic reaction) of the metal dissolving as ions generates some electrons,
as shown in the simple model on the left, that are consumed by a secondary process (cathodic reaction).
These two processes have to balance their charges.
The sites hosting these two processes can be located close to each other on the metal's surface,
or far apart depending on the circumstances.
The electrons (e-) produced by the corrosion reaction will need to be consumed by a cathodic reaction
in close proximity to the corrosion reaction itself.
The electrons and the hydrogen ions react first to form atomic hydrogen, and subsequently molecular hydrogen.
If the acid level is high (low pH), this molecular hydrogen will readily escape as a gas or may react with
dissolved oxygen, converting it into water.
Corrosion accelerating effects include high concentrations of free hydrogen ions (low pH), which speeds the release of electrons
and high water temperatures, which increase virtually all chemical reaction rates.
Thus a variety of natural and environmental factor can have significant effects on the corrosion rate of metals.
Metal oxidation in the most common use of the word, means electrochemical oxidation of the
metal in reaction with an oxidant such as oxygen or sulphur.
Rusting, the formation of iron oxides, is a well-known example of electrochemical corrosion.
This type of disintegration typically produces oxide(s) or salt(s) of the original metal, and results
in a distinctive orange colouration.
Corrosion degrades the useful properties of materials and structures including strength, appearance.
Many structural alloys corrode merely from exposure to moisture in air, but the process
can be strongly affected by exposure to certain substances.
Corrosion can be concentrated locally to form a pit or crack, or it can extend across a
wide area more or less uniformly corroding the surface.
Because corrosion is a diffusion-controlled process, it occurs on exposed surfaces.
As a result, methods to reduce the activity of the exposed surface, such as passivation
and chromate conversion, can increase a material's corrosion resistance.
However, some corrosion mechanisms are less visible and less predictable.
Galvanic corrosion
Galvanic corrosion also called bimetallic corrosion is an electrochemical process named after
Luigi Galvani (1737 – 1798),
in which one metal corrodes preferentially when it is in electrical contact with
another, in the presence of an electrolyte.
A similar galvanic reaction is exploited in primary cells or batteries to generate a useful
electrical voltage to power portable devices.
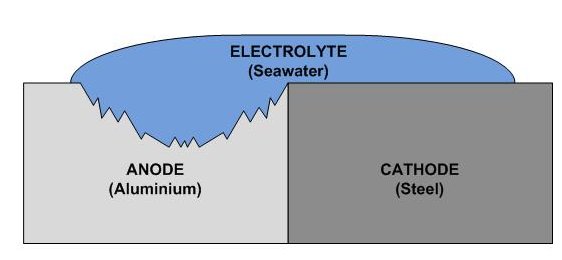
When two metals are submerged in an electrolyte (e.g. seawater), while also
electrically connected (e.g. by an external conductor or direct contact),
the less noble metal (anode) will experience galvanic corrosion.
The electro-potential difference between the reactions at the two electrodes is
the driving force for an accelerated attack on the anode metal, which dissolves
into the electrolyte.
This leads to the metal at the anode corroding more quickly than it otherwise
would and corrosion at the cathode side being inhibited.
The presence of an electrolyte and an electrical conducting path between the metals
is essential for galvanic corrosion to occur.
The electrolyte provides a means for ion migration whereby ions move to prevent
charge build-up that would otherwise stop the reaction.
The rate of corrosion is determined by the electrolyte, the difference in nobility,
and the relative areas of the anode and cathode exposed to the electrolyte.
The difference in nobility can be measured as a difference in voltage potential:
the less noble metal is the one with a lower (more negative) electrode
potential than the nobler one, and will function as the anode (electron or anion
attractor) within the electrolyte device. For example the voltage difference between
a copper plate and a piece of galvanized steel (zinc covered) in fresh water
is about 0.8V with the galvanized steel more negative than the copper part of the
galvanic pair.
The galvanic series (or electro-potential series)
lists the order of nobility of metals and semi-metals and provides the difference in
electro-potential. It can be used to determine how a galvanic pair of metals will behave.
For galvanic corrosion to occur, three condition must be fulfilled:
two different metals - must be electrically connected - and both must be (at least partialy) submerged in a conducting electrolyte
|  |
Given these conditions, there are several ways of reducing and preventing this form of corrosion.
Electrically insulate the two metals from each other.
If they are not in electrical contact, no galvanic coupling will occur.
This can be achieved by using non-conductive materials between metals of different electro-potential. Ensure there is no electrical contact with an electrolyte.
This can be done by using water-repellent compounds such as greases, or by coating
the metals with an impermeable protective layer, such as a suitable paint,
varnish, or plastic.
If it is not possible to coat both, the coating should be applied to the more noble material (cathode).
This is advisable because if the coating is applied only on the more active material (anode),
in case of damage to the coating there will be a large cathode area and a small anode area,
and for the exposed anodic area the corrosion rate will be correspondingly high. Using antioxidant paste is beneficial for preventing corrosion
between copper and aluminium electrical connections. The paste consists of a lower
nobility metal than aluminium or copper (e.g. zinc). Choose metals that have similar electro-potentials.
The more closely matched the individual potentials, the lesser the potential difference
and hence the lesser the galvanic current. Using the same metal for all construction
is the easiest way of matching potentials (e.g. stainless steel for the complete rigging). Electroplating or other plating can also help. This tends to
use more noble metals that resist corrosion better. Chrome, nickel, silver and gold
can all be used to protect the less noble base metal. Another approach is galvanizing with zinc,
which protects the steel base metal by sacrificial anodic action of the plating layer. Cathodic protection uses one or more sacrificial anodes made
of a metal which is more active than the protected metal. Alloys of metals commonly
used for sacrificial anodes include zinc, magnesium, and aluminium. Metal boats connected to a shore line electrical power feed will
normally have to have the hull connected to the supply ground for safety reasons.
However the physical end of that ground connection is likely to be a copper
rod buried within the marina, resulting in a steel-copper "battery" of about 0.5V potential.
For such cases, the use of a galvanic isolator is essential, typically two semiconductor
diodes in series, in parallel with two diodes conducting in the opposite direction.
This prevents any current while the applied voltage is less than 1.4 V (i.e. 0.7 V per diode),
but allows a full current in case of an electrical fault.
There will still be a very minor leakage of current through the diodes, which may
result in slightly faster corrosion than normal.
Stray current corrosion
Stray current corrosion is different from galvanic corrosion in that
electricity from an outside source flows through immersed metal structures
(e.g. the vessel's grounding systems), whereas galvanic corrosion is caused
by the inherent electrical potential of dissimilar connected metals.
The currents responsible for this stray corrent corrosion may come from
DC distribution lines, railway systems, defectively grounded AC power lines
and many other infrastructural sources.
Seeking the path of least resistance, the stray current from a foreign installation may
travel preferably along a vessel's grounding system causing severe local corrosion
on the electrically positive side of the stray path (at the anode).
The metal on the negative side (the cathode) will not be affected by stray current corrosion.
The process of stray current corrosion is electrolysis in nature.
The extent of damage or loss of metal is directly proportional to the magnitude of stray
current passing through the system.
Stray current corrosion tends to be localized, causing a concentration of pits that is not
normally observed at locations where the stray current leaves metal structure (anode).
Stray current strengths may be much higher than those produced by galvanic cells and,
as a consequence, corrosion may be much more rapid than in natural deterioration processes.
Another difference between galvanic-type currents and stray currents is that the latter
are more likely to operate over long distances since the anode and cathode are more likely
to be remotely separated from one another.
The knowledge of the presence of stray currents becomes highly important when remedial measures
are undertaken since a simple sacrificial anode system is likely to be quite ineffectual
in preventing corrosion under most circumstances.
Instead, the grounding of the vessel must be carefully designed to prevent stray current
from going through the vessel's grounding system.
See also the Note on Stray Current Corrosion.
|
 Corrosion is the primary means by which metals deteriorate.
Most metals corrode on contact with water (and moisture in the air),
acids, bases, salts, oils, aggressive metal polishes, and other solid
and liquid chemicals.
Metals will also corrode when exposed to gaseous materials like acid vapours,
formaldehyde gas, ammonia gas, and sulphur containing gases.
Corrosion specifically refers to any process involving the deterioration
or degradation of metal components. The best known case is that of the
rusting of steel.
Corrosion is the primary means by which metals deteriorate.
Most metals corrode on contact with water (and moisture in the air),
acids, bases, salts, oils, aggressive metal polishes, and other solid
and liquid chemicals.
Metals will also corrode when exposed to gaseous materials like acid vapours,
formaldehyde gas, ammonia gas, and sulphur containing gases.
Corrosion specifically refers to any process involving the deterioration
or degradation of metal components. The best known case is that of the
rusting of steel.