Galvanic Series
With the galvanic series, metals are grouped according to electrical potential
measured to a known reference.
This galvanic series (or electro-potential series) determines the nobility of
metals and semi-metals. In a situation in which galvanic corrosion will
occur with two dissimilar metals, the less noble metal will be depleted
and physically "dissolved" in the electrolyte. The nobler of the involved
metals will remain intact and will not suffer from the corrosion process. Galvanic Table for SeawaterThe following galvanic table lists metals in the order of their relative activity in seawater environment. On top of the list are the more active (anodic) metals and proceeding down are the least active (cathodic) metals of the galvanic series. A galvanic series applies to a particular electrolyte solution, hence for each specific solution, which is expected to be encountered for actual use, a different order or series will ensue. If combined in a galvanic couple, the metal higher in the series (or the smaller voltage potential) represents the anode, and will corrode preferentially in the specific environment. 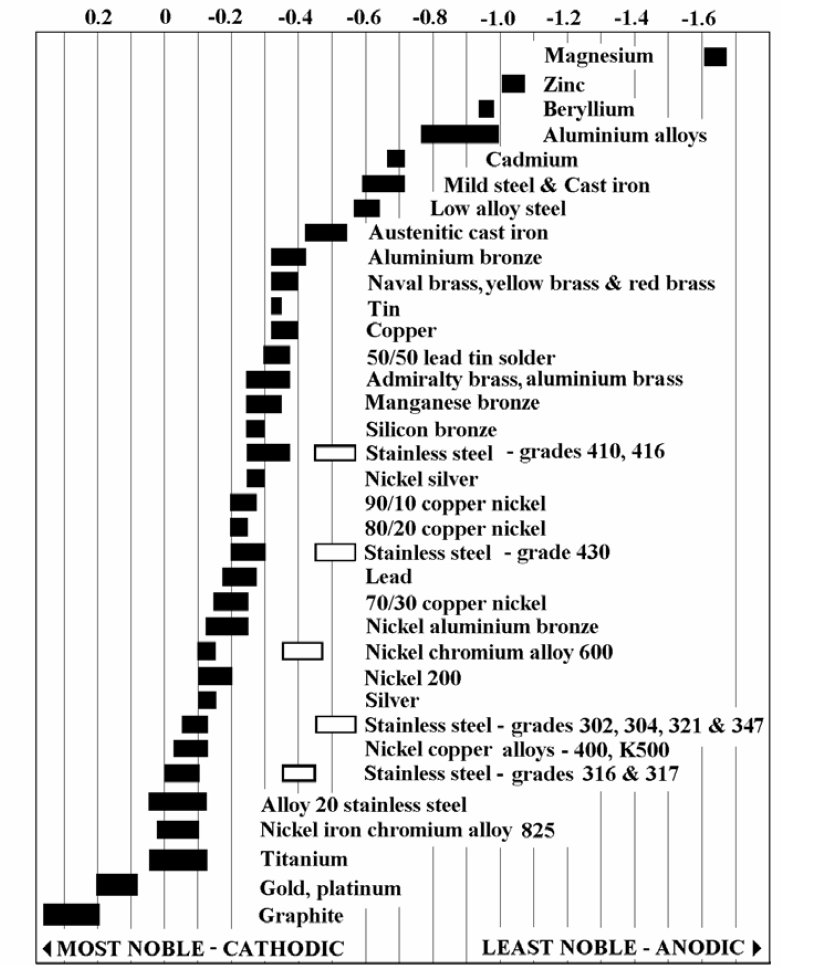
The chart above shows corrosion potentials in flowing seawater at ambient temperature.
The unshaded symbols show the ranges exhibited by
stainless steels in acidic water
such as may exist in crevices or in stagnant, low velocity or poorly aerated water.
Notice the shift in voltage potential due to the change of the electrolytic environment. |
| Cover << Sail Away << Corrosion << . | . >> Sacrificial Anodes | last updated: 22-Mar-2018 |