Batteries - a short History
Ancient galvanic cells have been discovered in Sumerian ruins outside of
Baghdad dated to around 250 B.C..
The cells were composed of a clay jar with a iron rod surrounded by a
copper cylinder and a vinegar as electrolyte.
Asphalt was used to seal the jar and to fixate the electrodes.
These first galvanic cells were probably used for electroplating jewellery
with thin layers of gold or silver.
The re-discovery of the battery in Europe was demonstrated more than 2000
years later.
Most historians date the invention of batteries to about 1800 when
experiments by Alessandro Volta resulted in the generation of electrical
current from chemical reactions between dissimilar metals.
The original voltaic pile used zinc and silver disks and a separator
consisting of a porous non-conducting material saturated with sea water.
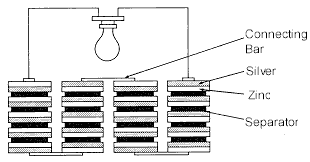 When stacked, a voltage could be measured across each silver and zinc disk.
Experiments with different combinations of metals and electrolytes continued
over the next 60 years.
When stacked, a voltage could be measured across each silver and zinc disk.
Experiments with different combinations of metals and electrolytes continued
over the next 60 years.
Large and bulky variations of the voltaic pile provided the only practical source of
electricity in the early 19th century.
They were the original primary battery.
Johann Ritter first demonstrated the elements of a rechargeable battery in 1802,
but rechargeable batteries remained a laboratory curiosity until the development,
much later in the century of practical steam-driven dynamos to recharge them.
During the first half of the 19th century experiments continued with a variety of
electrochemical couples (combinations of positive and negative electrode materials
and electrolyte).
Finally about 1860, the ancestors of today's primary and secondary batteries were
developed.
On the primary side, in the 1860's George Leclanché developed a first form
of the carbon-zinc battery.
The original version was a wet cell with the electrodes immersed in a pool of
electrolyte.
It became popular because it was rugged, easy to manufacture, and stored well.
Secondary batteries date back to 1860 when Raymond Gaston Planté invented
the lead-acid battery.
His cell used two thin lead plates separated by rubber sheets.
He rolled up the combination and immersed it in a dilute sulphuric acid solution.
Initial capacity was extremely limited since the positive plate had little active
material available for reaction.
About 1881, Fauré and others developed batteries using a paste of lead oxides
(PbO2) for the positive plate active materials.
This allowed much quicker formation and better plate efficiency than the solid
Planté plate.
Although the rudiments of the flooded lead-acid battery date back
to the 1880's, there has been a continuing stream of improvements
in the materials of construction and the manufacturing and
formation processes.
Since many of the problems with flooded lead-acid batteries involved
electrolyte leakage, many attempts have been made to eliminate
free acid in the battery.
German researchers developed the gelled-electrolyte lead-acid
battery in the early 1960's which was a major improvement.
Todays high performance gel-cell batteries are based on this
principle.
Since they contain no water-based electrolyte, they can also
be used upside-down, which make them attractive for usage
on vessels.
|
 When stacked, a voltage could be measured across each silver and zinc disk.
Experiments with different combinations of metals and electrolytes continued
over the next 60 years.
When stacked, a voltage could be measured across each silver and zinc disk.
Experiments with different combinations of metals and electrolytes continued
over the next 60 years.