Principle of Operation
The watermaker installation is based on a process called reverse osmosis and can be
seen a a very fine filter that is capable of blocking salt and other dissolved ions.
In order to pass the sea water through the fine membrane filter, a high water pressure
of over 50 bar is required.
Therefore, besides the membrane filter a high-pressure pump is the key element in a
watermaker installation.
The pump can be driven directly from the propulsion engine of from an extra electrical
pump motor.
Osmosis
Osmosis was first described as a process observed in biological cell membranes.
It is an essential aspect in biological systems, as biological membranes are semi-permeable.
In general, these membranes are impermeable to large and polar molecules, such as ions, proteins,
and polysaccharides, while being permeable to non-polar and hydrophobic molecules like lipids
as well as to small molecules like oxygen, carbon dioxide, nitrogen, nitric oxide, etc.
Osmosis is a type of diffusion which can occur when there is a partially permeable membrane,
such as a cell membrane.
When a cell is submerged in water, the water molecules pass through the cell membrane from
the side of low solute concentration to the side of high solute concentration.
So the direction of the water flow depends on the difference of concentration inside and
outside of the cell:
if the cell is submerged in salt water, water molecules move out; if it is submerged
in freshwater, however, water molecules will move into the cell.
In biological cells, the cell membranes are selectively permeable, so only the necessary
materials are let into the cell and wastes are left out.
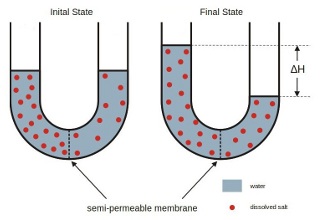 The word 'osmosis' is particular to the diffusion of water molecules through a semi-permeable membrane.
When the membrane has a volume of pure water on both sides, water molecules pass in and out
in each direction at exactly the same rate; there is no net flow of water through the membrane.
The word 'osmosis' is particular to the diffusion of water molecules through a semi-permeable membrane.
When the membrane has a volume of pure water on both sides, water molecules pass in and out
in each direction at exactly the same rate; there is no net flow of water through the membrane.
In the case of water with different salinities such as sea water and drinking water,
the following can be observed: if water mixtures with different salinities are
separated by a semi-permeable membrane, the mixtures tend to equalize their salt concentration
by forcing a transport of water molecules from the low salinity side to the high salinity side
of the membrane.
This forced transport of water molecules through the semi-permeable membrane is driven by
the osmotic pressure caused by the initial difference in concentration of dissolved components.
For salt water concentration this osmotic pressure is in the order of magnitude of 30 bar!
The principle of reverse osmosis
Osmosis is the directed migration of molecules through a semi permeable membrane.
The chemical and physical structure of the membrane determines which molecules are
able to pass and which are not.
For this reason, it is called semi-permeable, which means as much as halfway or
partial permeability.
 The effect would be a less concentrated, homogeneous dissolution.
Pouring sea- and fresh water in equal amounts into a container where both liquids
are separated by an adequate semi-permeable membrane, there would be one side with
seawater that is highly loaded with salts, on the other side more or less “clean”
water without or with little dissolved components.
The effect would be a less concentrated, homogeneous dissolution.
Pouring sea- and fresh water in equal amounts into a container where both liquids
are separated by an adequate semi-permeable membrane, there would be one side with
seawater that is highly loaded with salts, on the other side more or less “clean”
water without or with little dissolved components.
The natural tendency of both liquids to equalize their salinity leads to the migration of
water molecules from the fresh water side towards the seawater side. As a result, the
volume of water on the fresh water side decreases while it increases on the seawater
side. This process of osmosis takes place until the pressure on the seawater side is
in accordance with the osmotic pressure.
Then it stops. In this case, the osmotic pressure is around 30 bar.
The described process however is reversible by exposing the liquid on the seawater side
to mechanical pressure.
At a pressure of 30 bar, the osmotic process cannot take place or would rather be reversed.
When pressure is increased beyond 30 bar, for instance 60 bar, water molecules from the seawater
side migrate to the fresh water side.
All other components of the seawater dissolution are not able to pass the membrane.
As a result, the dissolution on the seawater side remains highly-concentrated while
there is a gain of fresh water on the other side of the membrane.
This process is referred to as reverse osmosis (R.O.)
The seawater is delivered to the pressure pipe’s entry side of a RO-system by a high-
pressure pump. The osmotic membrane is located in the pressure pipe and is merely
permeable for the vehicle “water“ (solvent) and detaining the soluted substances.
When the pressure difference is more than levelling out the osmotic head, the water
molecules are able to pass the membrane that works like a filter, while the
“unpurified” molecules are detained. In opposite to a classic membrane-filter,
osmosis membranes do not have continuous pores. In fact, the ions and molecules
are migrating through the membrane by diffusing through the membrane material.
Inside of the membrane, the permeat-tube is located which transports the fresh water
through one of the two end caps of the pressure pipe towards the fresh water tank.
The excess seawater, now referred to as concentrate (brine), is drained off
overboard through the outlet of the pressure pipe by a pressure control valve.
Is watermaker product safe to drink?
Provided the membrane element is in good condition, it “filters” to about 0.0005 micron.
Virus (0.01 to 0.04 micron) or bacteria (0.1 to 15 micron) will not pass and in reality,
water-borne diseases on board vessels using only desalinated water are un-heard of.
However, the condition of the vessels storage tank(s) and/or plumbing can be a concern
and should be sterilized e.g: with chlorine on commissioning of the desalination system
and as an occasional routine maintenance.
The desalination system should not be operated in chemically contaminated waters as the
RO process will not safely remove all chemicals.
Active carbon filters after the tank and before the faucet are useful to remove chlorine
from shore water but do not improve the watermaker product quality.
What is the effect of seawater temperature on the freshwater production rate?
The freshwater output of most desalination systems is rated at a seawater feed temperature
of 26˚Celsius. Membrane productivity, however, is sensitive to changes in feedwater temperature.
As water temperature changes, water flux changes almost linearly, due primarily to the
diffusion rate of water through the RO membrane.
As a rule of thumb, membrane capacity changes about 2% per degree Celsius water temperature.
At very low feed temperatures, pre-heating of the feed water may be considered.
A commonly used method of pre-heating is mixing part of the engines/generators seawater
cooling return with the cold seawater, ideally to a temperature of 30˚ Celsius.
|
 The word 'osmosis' is particular to the diffusion of water molecules through a semi-permeable membrane.
When the membrane has a volume of pure water on both sides, water molecules pass in and out
in each direction at exactly the same rate; there is no net flow of water through the membrane.
The word 'osmosis' is particular to the diffusion of water molecules through a semi-permeable membrane.
When the membrane has a volume of pure water on both sides, water molecules pass in and out
in each direction at exactly the same rate; there is no net flow of water through the membrane. The effect would be a less concentrated, homogeneous dissolution.
Pouring sea- and fresh water in equal amounts into a container where both liquids
are separated by an adequate semi-permeable membrane, there would be one side with
seawater that is highly loaded with salts, on the other side more or less “clean”
water without or with little dissolved components.
The effect would be a less concentrated, homogeneous dissolution.
Pouring sea- and fresh water in equal amounts into a container where both liquids
are separated by an adequate semi-permeable membrane, there would be one side with
seawater that is highly loaded with salts, on the other side more or less “clean”
water without or with little dissolved components.